All Things Consist of Atoms
Basic knowledge about atoms is beneficial for comprehending electricity. Atoms serve as the fundamental constituents of the cosmos. All entities inside the universe, including stars, trees, and animals, are composed of atoms. Atoms constitute the human body. Both air and water consist of atoms. Atoms are of such minute size that millions of them might be accommodated on the surface of a pinhead.
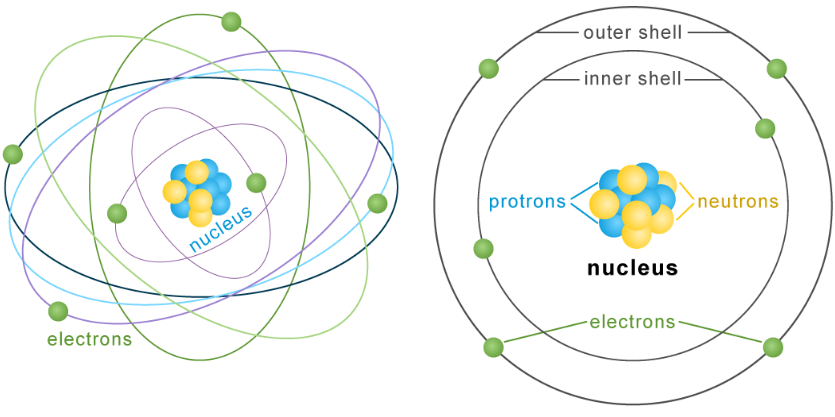
Atoms Consist of Minuscule Particles
The nucleus is the central region of an atom. The nucleus consists of protons and neutrons, which are particles. Electrons orbit the nucleus within energy shells. If the size of the nucleus were comparable to that of a tennis ball, the atom would possess dimensions equivalent to a sphere with a diameter of around 1,450 feet, or roughly equivalent to the dimensions of one of the most expansive sports stadiums globally. Atoms primarily consist of voids.
If an atom were visible to the unaided human sight, it would exhibit a resemblance to a minute aggregation of spherical entities encompassed by colossal imperceptible voids (or encapsulations). The electrons would reside on the surface of the bubbles, perpetually rotating and relocating to maintain maximum distance from one another. Electrons are captured within their shells using an electrical force.
The protons and electrons within an atom exhibit mutual attraction. Both of these entities possess an electrical charge. Protons possess an electrical charge of +, while electrons possess an electrical charge of -. The proton’s positive charge is equivalent to the electron’s negative charge. Charges that are opposite in nature exhibit an attractive force towards one another. Atomic equilibrium occurs when the number of protons and electrons in an atom is equal. Neutrons are electrically neutral and their quantity can fluctuate.
The classification of an atom, or element, is determined by the quantity of protons it possesses. An element can be defined as a substance that is composed of a singular sort of atom. The Periodic Table of Elements displays the atomic numbers of elements, which represent the quantity of protons they possess. As an illustration, it may be observed that each hydrogen (H) atom possesses a single proton, but each carbon (C) atom possesses six protons.
The Phenomenon of Electricity Involves the Transfer of Electrons between Atoms
Typically, electrons maintain a consistent distance from the nucleus of an atom within specific shells. The outermost shell of the nucleus has the capacity to accommodate two electrons. The subsequent shell has a maximum capacity of eight. The capacity of the outer shells can be further increased. Certain atoms possessing a large number of protons have the capacity to accommodate up to seven electron-occupied shells.